A new study by Mike Callahan, an associate professor in the Department of Chemistry and Biochemistry at Boise State, and Jim Cleaves, a chemistry professor at Howard University in Washington, D.C., may help answer one of the longstanding paradoxes in origin of life chemistry.
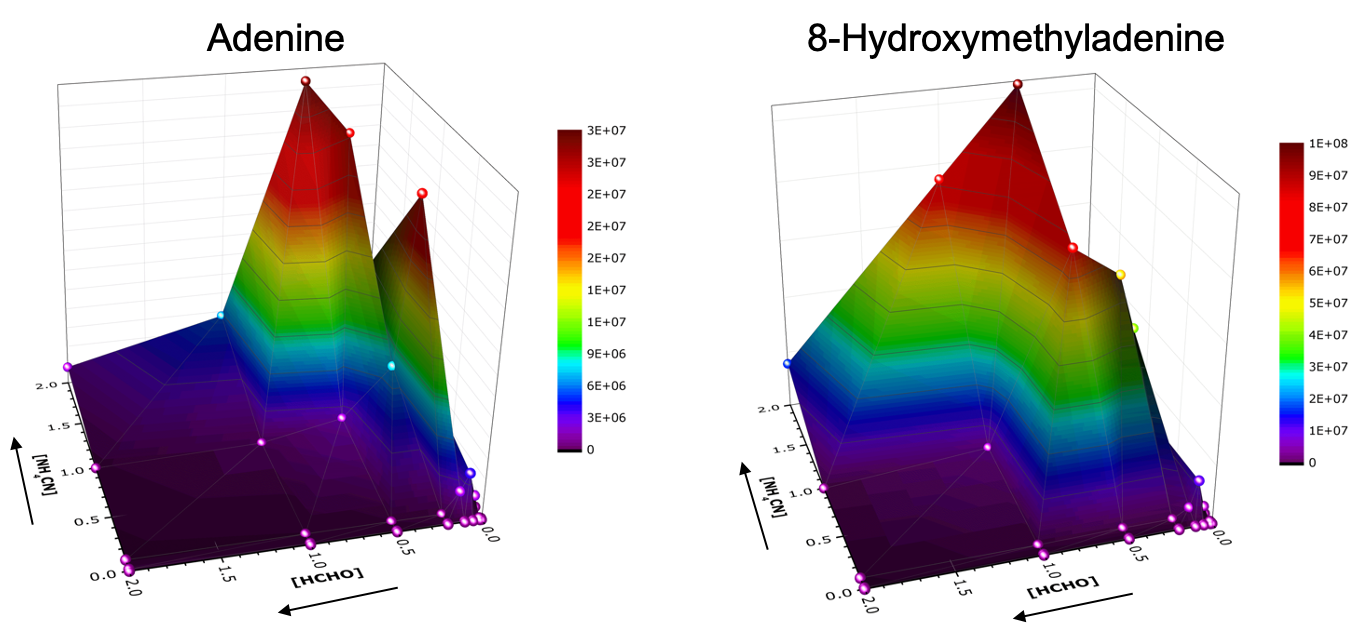
Billions of years ago, nucleobases may have emerged from reactions involving hydrogen cyanide. Sugars might have emerged from reactions involving formaldehyde. Both nucleobases and sugars are thought to be essential to the origin of life since they are building blocks of RNA and DNA. However, there is a longstanding belief that nucleobases and sugars cannot be synthesized in the same location since solutions containing both hydrogen cyanide and formaldehyde lead to the production of another compound (glycolonitrile), and would inhibit the synthesis of nucleobases and sugars. This scenario is often referred to as the Miller Paradox.

In the laboratory, Callahan and Cleaves prepared a large set of samples containing different concentrations of hydrogen cyanide and formaldehyde. Since the reaction is relatively slow, these samples sat in glass ampules for over a year before being analyzed by a high-resolution mass spectrometer. The team reported that nucleobases were synthesized under a wide range of hydrogen cyanide and formaldehyde concentrations. The synthesis of nucleobases was not inhibited at high formaldehyde concentration, but rather these nucleobases were modified by formaldehyde.
Their results are significant since the synthesis of both nucleobases and sugars could probably have occurred in the same geological setting. Ultimately, the Miller Paradox may not be as detrimental to synthesis as once thought.
“I believe that this study is an important contribution to the origin of life field. I think it will help change a narrative that has been engrained in a lot of scientists for many decades now,” Callahan said.
The research was funded by the NASA Emerging Worlds program, and was published in the journal ACS Earth and Space Chemistry.