Article by Graduate Research Assistant Simon Roy and Research Program Manager John Hall.
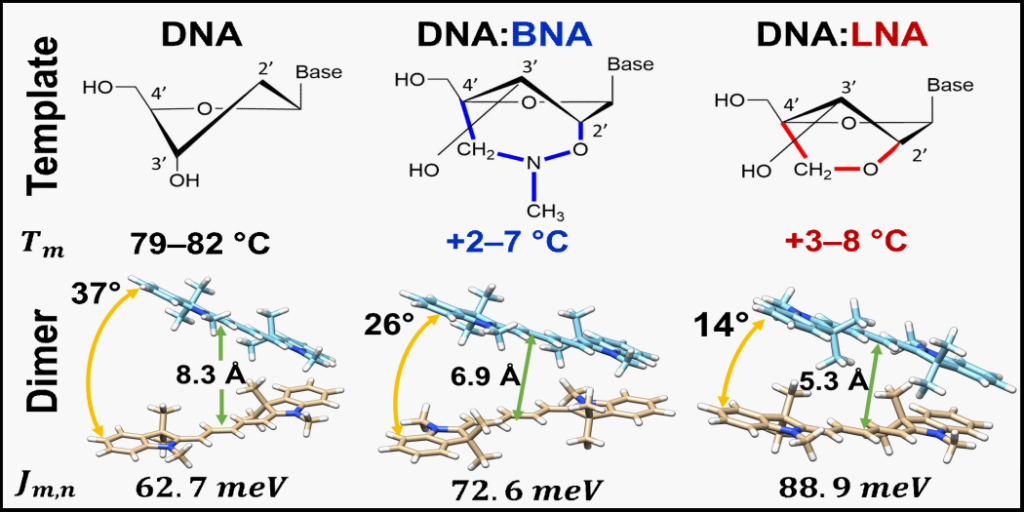
Bridged nulceic acids increase the stability of DNA scaffolds carrying cyanine dye Cy5 aggregates. In addition, the presence of bridged nucleic acids in the scaffold increase the coupling strength between the dyes and further enhance their face-to-face stacking arrangement.
What did the Scientists/ Engineers Discover?
Molecular excitons–or the bound state of an electron and its associated electron hole–arise from the collective interactions among coupled chromophores (dyes) that allows creation of excited states that are shared across participating dyes. The shared excitation energy extends in a wave-like manner over a closely spaced and oriented collection (aggregate) of two or more dyes (i.e., the energy is delocalized). Excitons have garnered considerable research interest arising from their function in natural light-harvesting systems and their potential applications in artificial light harvesting, organic optoelectronics, and nanoscale computing. In addition, deoxyribonucleic acid (DNA)-based nanotechnology has emerged as an effective and accessible method to design and control scaffolded dye aggregate systems at sub-nanometer scales.
One significant challenge in designing the DNA scaffold is the instability in dye proximity and orientations due to the dynamic nature of the DNA scaffold itself (so-called DNA “breathing”). Although many studies have documented changes in the optical properties of dyes upon aggregation using DNA scaffolds, the study by Quantum DNA Research Group member and MSMSE PhD student Simon Roy and colleagues reported here represents a mostly unique investigation regarding how structural modifications of DNA via bridged nucleotides and dye attachment method can impact scaffold stability, as well as the configuration and optical behavior of attached aggregates. Using a widely available commercial dye, cyanine dye Cy5, Roy and colleagues demonstrated that the presence of bridged nucleic acids in the scaffold not only increased the stability of the scaffold but also enhanced desired optical properties of the dye aggregates. A key component of this study was the utilization of modeling software developed here at Boise State by Dr. Bernard Yurke. Dr. Yurke’s program relates experimental observations to the quantum mechanical theory that governs dye-aggregate systems. The software attempts to predict the relative orientations of the dyes and a number of other key system properties to gain insight into interactions between dye molecules.
Impact
The study by Roy and colleagues demonstrated the feasibility and utility of incorporating bridged nucleic acids into a DNA scaffold used to control the proximity and orientation of dye aggregates and thereby obtaining and enhancing desired optical properties. The approach helps to mitigate one of the current drawbacks of using DNA as a scaffold–that is the dynamic nature of DNA itself.
Study Investigators
* All investigators below are affiliated with Boise State University
- Simon K. Roy, Micron School of Materials Science and Engineering (MSMSE)
- Olga A. Mass, MSMSE
- Donald L. Kellis, MSMSE
- Christopher K. Wilson, MSMSE
- John A. Hall, Division of Research and Economic Development
- Bernard Yurke, MSMSE and Department of Electrical and Computer Engineering (ECE)
- William B. Knowlton, MSMSE and ECE
Publication Citation
Roy, SK, OA Mass, DL Kellis, CK Wilson, JA Hall, B Yurke, and WB Knowlton, Exciton Delocalization and Scaffold Stability in Bridged Nucleotide-Substituted, DNA Duplex-Templated Cyanine Aggregates, Journal of Physical Chemistry B, 125, 13670-13684(2021). doi: 10.1021/acs.jpcb.1c07602.
Funding
| Funding Agency | Grant/Contract Number | Role of Funding |
|---|---|---|
| U.S. Department of Energy, Idaho National Laboratory, Laboratory Directed Research and Development project through Battelle Energy Alliance | Blanket master contract No. 154754 between Battelle Energy Alliance and Boise State University, Release 15 | Primary funding for Roy et al. 2021 |
| National Institutes of Health (NIH), MJ Murdock Charitable Trust, and Idaho State Board of Education (to the Biomolecular Research Center at Boise State) | NIH award Nos. P20GM103408 and P20GM109095 | Use of the circular dichroism spectrometer |
Disclaimer
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the Department of Energy, Idaho National Laboratory and Battelle Energy Alliance.