Recent policy changes requiring clinical trial applications to be submitted to FOAs that specifically allow clinical trials, first announced in fall of 2016, impact how all NIH applicants choose a FOA, whether you are submitting a clinical trial or not.
Over the last year, each NIH Institute and Center has been carefully evaluating its research funding priorities and strategic goals and using that information to articulate their funding priorities for clinical trials. They are communicating their priorities through the funding opportunity announcements they issue.
The requirement to respond to clinical trial specific FOAs begins for applications submitted for due dates on or after January 25, 2018. NIH is reissuing any FOA that will accept clinical trial applications after that date. Many of these FOAs have already been issued, others will be published at least 60 days before the first due date for which they will accept applications. How can you tell which FOAs will allow clinical trials? Reissued clinical trial FOAs make clinical trial allowability clear in both the title and in section 2, and they include clinical trial review criteria.
Responding to the correct type of FOA ensures that you know what information you are expected to include in your application and that you can develop an application that is responsive to the review criteria. It also ensures that reviewers apply the correct criteria and give your application the best review possible.
Before beginning your search for an FOA, if you are doing human subject research you should use our clinical trial tool to determine whether NIH considers any of your studies a clinical trial.
If any study (or component) of your application meets the NIH definition of a clinical trial (even if your application includes other studies that are not clinical trials), you must respond to a FOA that allows for clinical trials.
If none of your specific aims include studies that meet the NIH definition of a clinical trial, be sure to respond to an FOA that does not require clinical trials. Check section II of the FOA; there will be a row entitled “Clinical Trial?” that should say either “Clinical trials not allowed” or “Clinical trials optional”.
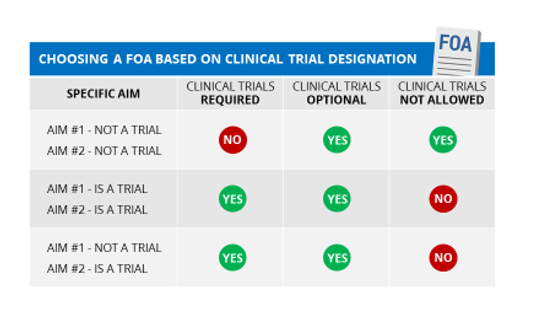
We are re-issuing existing parent announcements as “clinical trial not allowed” for due dates on or after January 25, 2018. Our most recent reminder notice provides a list of all the parent announcements (old and new) and when they will be reissued. .) . The participating organizations may vary between the “clinical trial not allowed” parent FOA and the “clinical trial required” parent FOA for the same activity code. Read the details of each FOA carefully. Note that some institutes that participate on a “Clinical Trial Required” parent may limit their participation to mechanistic studies. Check the Related Notices section of the FOA for any restrictions.
Some IC’s are using different FOAs for different kinds of trials. We encourage you to visit individual IC’s web pages for guidance.
Note that even for resubmissions, revisions or renewals, you may need to find a new FOA to apply to with the appropriate clinical trial allowability that reflects the research in the application you are submitting.
The upshot of all this? The FOA landscape is changing. It is important to pick your FOA carefully. We will be reissuing all parent FOAs and all FOAs that will allow clinical trials at least 60 days before the first due date. Before you are ready to apply, check back to be sure you are responding to the latest version of the FOA, and to read any related notices that have been issued since you first looked at the FOA. Learn more about understanding funding opportunities and NIH clinical trial requirements on the NIH Grants and Funding website. And be on the lookout for a new video we will be putting out in the next few weeks on finding and understanding funding opportunities.