Dilution Gauging
Goal
Compute the instantaneous discharge of a small stream using the slug injection method
Learning Objectives
Upon completion of this exercise students will be able to
- Understand why standard velocity-area methods are difficult to accomplish in small streams
- Understand the concepts underlying the dilution gauging method for measuring instantaneous discharge
- Collect data required for dilution gauging
- Compute discharge for slug and constant injection methods
Project Files
- Provide links to files students may need, not including data files they will discover on the websites.
Requirements and Connections
- List an prerequisite exercises students should complete
In small or highly turbulent streams it can be difficult to get accurate velocity measurements using standard velocity meters. An alternative approach is to introduce a chemical tracer, such as dissloved salt, to the stream and monitor its concentration at a point downstream. The “breakthrough” curve of the salt at a downstream location is directly related to the flow rate of the stream. A salt tracer can be introduced as a constant injection or as an instaneous slug. In the constant injection method, the tracer with a known concentration is added to the stream at a known flow rate. Once fully mixed, the concentration of the solute in the stream will be diluted from the original tracer concentration in direct proportion to ratio the injection rate to the flow rate of the stream. Although simple to compute, field methods for the constant injection method can be cumbersome. Alternatively, a slug injection can be performed.
A salt slug injection involves dissolving a known mass, m, of salt into a volume of water to create a salt tracer solution, and then dumping the tracer into the stream. As the salt slug moves downstream, it disperses and travels as a function of stream discharge. At a point downstream, the concentration of the salt through time can be observed and plotted (called a breakthrough curve). The breakthrough curve can be used to compute discharge by considering continuity equations for the volumetric flow rate of water (discharge) and the mass flow rate of the solute.
If the salt solution is added instantaneously to a stream, the total flow rate, QT, at a point downstream at any time, t, will be the sum of the stream flow rate, Qs, plus the flow rate of the added tracer solution, Qtr

(1)
Similarly, the total mass flow rate, MT, of the dissolved solute at any time at the downstream point will be the sum of the mass flow rate of the solute that was already in the stream, Ms, plus the mass flow rate of the tracer, Mtr.
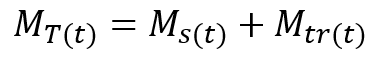
(2)
which can be rewritten as
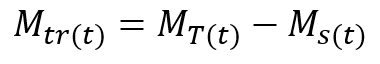
(3)
If no mass is “lost” between the upstream and downstream points, then the total mass of the solute in the tracer, mtr can be found by integrating equation 3 over the entire duration, D, that it takes for the mass to pass the downstream point
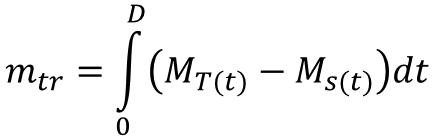
(4)
Mass flow rate is the product solute concentration and discharge. Equation 4 can therefore be written as
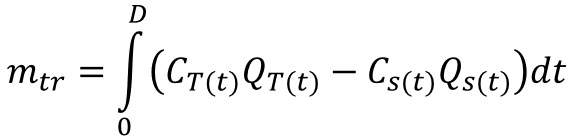
(5)
Considering equation 1, if we assume that the water flow rate of the tracer passing the downstream point at any time is negligible compared to the discharge of the stream, we can assume that the total flow rate is approximately equal to the stream flow rate. Substituting Qs for QT in equation 5, and assuming that Qs and Cs are constant produces
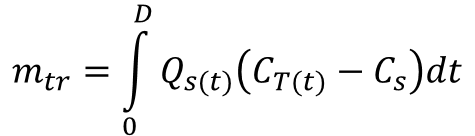
(6)
and Qs can be computed as
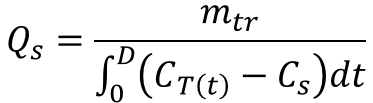
(7)
Because concentrations are measured in discrete time intervals, equation 7 is approximated as
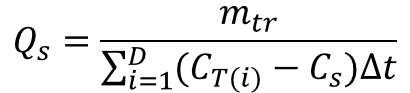
(8)
where CT(i) is the concentration of the solute in the stream at any time instant, i, separated by a time interval, Δt, between i=0 and the duration, D, of the breakthrough curve.
Because it is difficult to measure actual concentrations of solutes in the field, it is common to measure Electrical Conductivity (EC) instead. EC is a measure of a solutions ability to carry an electrical current, which is a function of the total dissolved solids in solution. We can relate solute concentration to EC through a calibration parameter, k.
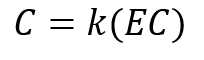
(9)
Stream flow rate can be computed using an EC breakthrough curve as
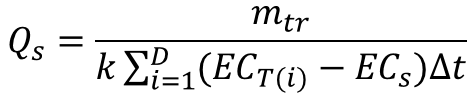
(10)
The parameter k must be obtained by calibration. This is best performed in the field prior to tracer injection. Field determination of k can be accomplished as follows:
- Collect a known volume of stream water in a bucket and measure its electrical conductivity (ECs).
- Add a known dry mass of salt to the bucket and let the salt dissolve completely. Compute the “relative concentration” (RC) as mass/volume. We use the term relative concentration because we do not know the background concentration of salt.
- After the solute is completely dissolved, measure and record the EC of the solution. (Note, it may not be possible to measure the EC of very high concentration solutions with standard field EC meters. Use serial dilution methods.)
- Add incremental amounts of salt to the bucket and stir to promote complete mixing. After each addition, compute the new relative concentration and and measure the EC.
- Construct a plot of RC (y-axis) versus EC (x-axis). Fit a linear equation to the relationship. The slope of the linear equation is k.
The instructions below allow students to compute discharge using the slug dilution gauging method using previously collected field data.
The Bogus South stream gauging site drains a small alpine watershed in the highest elevations of the DCEW. The stream bed is rocky and shallow such that gauging with the velocity-area method is often not possible. In 2011 a weir was installed to monitor discharge. However, at very low flows the weir loses accuracy. Slug injection dilution gauging is often performed to measure discharge at this site.
The excel file provides data for a dilution gauging measurement conducted on April 9, 2009. 92 g of Sodium Bromide was added to 18.4 liters of water to create a tracer solution. Once dissolved, the tracer was dumped into the stream approximately 50 m upstream of a monitoring location where electrical conductivity was measured once every three seconds. Prior to the injection, a field calibration was performed to compute the calibration parameter k (see background). The steps below guide students through process of computing discharge.
- Compute the calibration parameter, k. 9.2 liters of stream water were collected in a clean plastic bucket. The EC of this water was measured as 102 uS/cm. A relationship between relative concentration and EC was then constructed by adding incremental masses of NaBr to the water, computing the relative concentration (RC) (see background), and measuring the EC (Table 1).
- Compute the relative concentration for each mass NaBr added in cell under heading RCkBr (mg/l) of Table 1.
- A plot of RC versus EC is automatically generated. Fit a linear trendline to the data and obtain the slope of the linear equation. You will use this slope as k in Equation 10. Record the slope in Table 3.
- Compute the denominator of Equation 10.Columns A and B in Table 2 contain data to construct the solute breakthrough curve at the downstream monitoring location. Columns C-D, contain headings that illustrate steps to compute the denominator of Equation 10.
- Construct a plot of ECT versus Clock Time.
- Inspect the plot for data quality. We like to see a peak ECT of 3 times greater the ECs
- Compute the time interval in seconds in each cell of Column C. Note that the Clock Time unit is days
- For each cell in column D, compute the product of the time interval and the difference between ECT and ECs.
- Compute discharge, Qs.
- Enter mtr and ECs data in the appropriate yellow cells in Table 3.
- Compute the denominator of Equation 10 in the appropriate cell.
- Use equation 10 to compute Qs in the appropriate cell.
- Discussion and Analysis
- Look up the measured discharge value for the Bogus South site on April 9, 2009. How well does the slug injection data match the hydrograph data?
- An assumption of the dilution gauging method is that all of the injected mass passes the observation point. What are some situations that would cause this assumption to be violated?