Denise Wingett, Ph.D.

Professor, Department of Biological Sciences,
Director, Biomolecular Sciences Ph.D. Program
Year arrived at BSU: 2003
Mailing Address:
Department of Biology
Boise State University
Boise, ID 83725-1515
Office Location: Science Building, Room 103
Office Number: 208-426-2921
Office Fax: 208-426-1040
E-Mail Address: denisewingett@boisestate.edu
ACADEMIC DEGREES
B.S., Boise State University, Chemistry, 1986
Ph.D., Washington State University, Biophysics and Biochemistry, 1991
Postdoctoral Fellow, Washington State University, Microbiology Dept., 1992-1994
Research Assistant Professor, Neuroimmunology Dept., Portland VA Medical Center/Oregon Health Sciences University, 1994-1995
Medical Research Scientist, Boise VA Medical Center, 1998-2003
Affiliate Faculty, Div. of Gerontology and Geriatric Medicine, Univ. Washington School of Medicine, 2006-present
TEACHING
BIOL 297 Introduction to Bioinformatics
BIOL 310 Cell Biology
BIOL 420/520G Immunology
BIOL 446/546 Bioinformatics
BIOL 466/566 Advanced topics in Cancer and Immunology
BIOL 497/597Advanced topics in Immunology
BIOL 498/598 Infection and Immunity
BIOL 497/597 Immunology Laboratory
BIOL/BMOL 514 Flow Cytometry Research Techniques
BMOL 516 Responsible Conduct in Research in the Biomolecular Sciences
BMOL 598 Biomolecular Sciences Seminar Series
BMOL 605 Current Scientific Literature
BMOL 607 Graduate Research Presentation
BMOL 615 Research in the Biomolecular Sciences
RESEARCH INTERESTS
IMMUNOLOGY, NANOBIOLOGY, AND THERAPUETIC INTERVENTIONS FOR HUMAN DISEASE
The overall focus of our laboratory investigates how the immune system is involved in the pathogenesis of human diseases, including asthma, autoimmunity and cancer, and novel ways to treat disease including nanotechnology based approaches.
 Asthma and beta-adrenergic agonists:
Asthma and beta-adrenergic agonists:
A long standing interest of our laboratory has been to determine the underlying basis for the negative side effects of a commonly used class of asthma medications. Although beta-adrenergic agonists are effective in relieving the symptoms of asthma, serious concerns regarding their safety have persisted for more than 20 years. Recently, the FDA halted a large-scale study designed to examine the safety of these medications due to serious and life-threatening exacerbations and placed a black box warning on these drugs. In an effort to understand the molecular basis for adverse side effects, we have determined that an important regulatory protein (CD40L) expressed on T cells of the immune system is abnormally regulated by these drugs. Beta-agonists, by increasing cAMP levels in the cell, can elevate the expression of CD40L protein on T cells from asthmatics. This response is predicted to promote the underlying inflammatory responses contributing to disease. Conversely, beta-agonists decrease the expression of CD40L in healthy subjects. The goal of our research is to determine the molecular and cellular mechanisms leading to dysregulated CD40L protein expression in asthma. These finding should further our understanding of disease mechanisms, therapeutic intervention, and potentially lead to the identification of at-risk populations for adverse drug response.
Nanotechnology and biomedical applications:
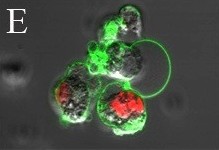
An active area of research in our lab is investigating the potential applications of metal oxidenanomaterials on the immune response and treatment of human diseases including cancer and autoimmunity. Nanoparticles with sizes in the 1 – 100 nm range,comparable to the sizes of naturally occurring biological molecules, are very attractive materials for manipulating biological structures and systems. When reduced to the nanoscale, many benign materials develop toxicity or other novel attributes, presumably due to the increased reactivity of the material surface. We have determined that different nanoparticle systems induce toxicity in a cell-specific and microenvironment-dependent manner. By controlling nanoparticle selectivity to specific cell types by design, we are currently exploring novel biomedical applications including cancer therapy and drug delivery.
Novel drug candidates and inflammatory disease:
We are investigating the potential utility of novel anthracycline drug analogs for the treatment of autoimmune diseases. The compound, DIDOX, belongs to a family of drugs called anthracyclines that are commonly prescribed for the treatment of cancer. Although anthracyclines have potent anti-proliferative effects that make them effective chemotherapeutic agents, a serious side effect of cardiotoxicity can develop 10-20 years later. A new drug analog, DIDOX, has been structurally modified to eliminate the molecular components associated with cardiotoxicity which opens the possibility of using this drug for the treatment of non-life threatening diseases such as psoriasis. We have determined that DIDOX effectively inhibits in vitro T cell responses as well as inflammatory responses in vivo. Future goals will be to evaluate the efficacy of this compound in animal models of psoriasis and other inflammatory diseases and to delineate the molecular mechanisms of immunosuppressive action.
RECENT PUBLICATIONS
Wyatt CR, Wingett D, White JS, Buck CD, Knowles D, Reeves D, and Magnuson NS: Persistent infection of rabbits with bovine leukemia virus associated with development of immune dysfunction. J Virol 63 :4498-4506, 1989.
Wingett D, Reeves R, and Magnuson NS: Stability changes in pim-1 proto-oncogene mRNA following mitogen stimulation of normal lymphocytes. J Immunol 147: 3563-3659, 1991.
Wingett D, Reeves R, and Magnuson NS: Characterization of the testes-specific Pim-1 transcript in rat. Nucleic Acids Res 20: 3183-3189, 1992.
Wingett D, Stone D, Davis WC, and Magnuson NS: Expression of the pim-1 proto-oncogene: Differential inducibility between alpha/beta and gamma/delta-T cells and B cells. Cell Immunol 162:123-130, 1995.
Wingett D, and Magnuson NS: Pim-1 proto-oncogene expression in anti CD3 mediated T cell activation is associated with protein kinase C activation and is independent of raf-1. J Immunol 156: 549-557, 1996.
Whitham RD, Wingett D, Wineman J, Mass M, Wegmann K, Vandenbark A, and Offner H: Treatment of relapsing autoimmune encephalomyelitis with T cell receptor V beta-specific antibodies when proteolipid protein is the autoantigen. J Neurosci Res 45: 104-116, 1996.
Wingett D, Forcier K, and Nielson CP: Glucocorticoid-medicated inhibition of RANTES expression in human T lymphocytes. Febs Lett 398:308-311, 1996.
Hoover DS, Wingett D, Zhang J, Reeves R, and Magnuson NS: Pim-1 protein expression is regulated by its 5’-untranslated region and translation initiation factor eIF-4E. Cell Growth Diff 8: 1371-1380, 1997.
Wingett D, Vestal RE, Forcier K, Hadjokas N, and Nielson CP: CD40 is functionally expressed on human breast carcinomas: Variable inducibility by cytokines and enhancement of Fas-mediated apoptosis. Breast Cancer Res Treat 50: 27-36, 1998.
Wingett D, Forcier K, and Nielson CP: Regulation of CD40L expression by cyclic AMP: Contrasting proinflammatory and inhibitory actions. Cell Immunol 192: 203-212, 1999.
Wingett D, Forcier K, and Nielson CP: A role for CD99 in T cell activation. Cell Immunol 193:17-23, 1999.
Nielson CP, and Wingett D: Endothelial cell and cAMP regulation of T cell CD40: Relevance of CaMKIV signaling. Immunol 105: 430-440, 2002.
Wingett D, and Nielson CP: Cyclic AMP differentially modulates CD40L expression on human naive and memory CD4+ T cells. Biochem Pharm 64: 1169-1178, 2002.
Wingett D, and Nielson CP: Divergence in NK cell and cyclic AMP regulation of T cell CD40L expression in asthmatic subjects. J Leuk Biol 74: 531-541, 2003.
Nielson C, and Wingett D: Intensive care and invasive ventilation in the elderly patient, implications of chronic lung disease and comorbidities. Chron Respir Dis 1: 43-54, 2004.
Matthies KGM, Hodzic A, and Wingett D: Differential regulation of soluble and membrane CD40L protein in T cells. Cell Immun 241: 47-58, 2006.
Olson RD, Headley MB, Walsh GM, and Wingett D: In vitro and in vivo immuno-suppressive activity of a novel anthracycline, 13-deoxy, 5-iminodoxorubicin. International Immunopharmacology, 7: 734-743, 2007.
Reddy KM, Feris K, Bell J, Wingett DG, Hanley C, and Punnoose A: Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Applied Physics Letters, 90: 213902, 2007.
Hanley C, Layne J, Punnoose A, Reddy KM, Coombs I, Coombs A, Feris K, and Wingett D: Preferential killing of cancer cells and activated human T cells using zinc oxide nanoparticles, Nanotechnology, 19: 295103, 2008.
Wang H, Wingett D, Engelhard ME, Feris K, Reddy KM, Turner P, Layne J, Hanley C, Bell J, Tenne D, Wang C, and Punnoose A: Fluorescent dye encapsulated ZnO particles with cell-specific toxicity for cancer treatment and bio-medical applications. Journal of Material Science: Materials in Medicine, 20: 11-22, 2009.
Hanley C, Thurber, A, Hanna C, Punnoose A, Zhang J, and Wingett D: The influences of cell type and ZnO nanoparticle size on immune cell cytotoxicity and cytokine induction. Nanoscale Research Letters, 4: 1409-1420, 2009.
Feris K, Otto C, Tinker J, Wingett D, Punnoose A, Thurber A, Quinn B, Hanna C, and Pink A: Electrostatic interactions affect nanoparticle-mediated toxicity to the Gram negative bacterium Pseudomonas aeruginosa PAO1, Langmuir, 26: 4429-4436, 2010.
Rasmussen JW, Martinez E, Louka P, and Wingett D: Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opinion on Drug Delivery, 7, 1063-1077, 2010.
Zhang J, Thurber A, Tenne D, Rasmussen JW, Wingett D, Hanna C, and Punnoose A: Enhanced dye fluorescence in novel dye-ZnO nano-composites. Advanced Functional Materials, 20: 4358,2010.
Thurber A, Wingett DG, Rasmussen JW, Layne J, Johnson L, Tenne DA, Zhang, J, Hanna CB, and Punnoose A: Improving the selective cancer killing ability of ZnO nanoparticles using Fe doping, Nanotoxicology, 6(4): 440-52, 2012.
Punnoose A, Dodge K, Rasmussen J, Chess J, Wingett D, and Anders C: Cytotoxicity of ZnO nanoparticles can be tailored by modifying their structure: A green chemistry approach for safer nanomaterials. ACS Sustainable Chemistry & Engineering, 2: 166-1673, 2014.
Anders C, Chess J, Wingett D, and Punnoose A: Serum proteins enhance solution stability and influence the cytotoxicity and dosimetry of ZnO nanoparticles in suspension and adherent cell models, Nanoscale Research Letters, 10: 448, 2015.
Wingett D, Louka P, Anders C, Zhang J, and Punnoose A: A role of ZnO nanoparticle electrostatic properties in cancer cell cytotoxicity, Nanotechnology, Science and Applications, 15(9): 29-45, 2016
PATENTS
Wingett D, Punnoose A, Reddy KM. Preferential killing of cancer cells and activated human T-cells using the selective toxicity of zinc oxide nanoparticles. Patent Pending. Patent No.: US8,187,638, May 29, 2012.
Wang H, Wingett D, Feris K, Kongara MR, Punnoose A. Fluorescent particles comprising nanoscale ZnO layer and exhibiting cell-specific toxicity. Patent No.: US 7,939,560 B2, 2011.
Graduate Students

Mark Headley 2004-2005, Immunomodulatory properties of a novel anthracycline drug analog, M.S. awarded 2005, Boise State University.
Began Doctoral Training, University of Washington 2005 – 2010. Post-doctoral fellow at University of California, San Francisco.
Kelli Matthies 2004-2006, Differential Regulation of Soluble and Membrane CD40L proteins in T cells, M.S. awarded 2006, Boise State University. 2006 Senior Associate Scientist at Amgen, California, United States.
Alma Hodzic 2005-2007, Molecular mechanisms controlling T cell CD40L gene expression: implications for asthma, M.S. awarded 2007, Boise State University. 2007 Scientist at Novartis, Switzerland.